Boditech Med has obtained the export license of four TDM products

- Acquiring export license for Vedolizumab(UC/Crohn’s disease) and Etanercept(RA) by the MFDS
- Expanding its product line to 14 TDM products with export license
- With diverse distribution channels, Boditech Med aims to be a market leader in the global TDM market, estimated to be worth $2.26 billion globally
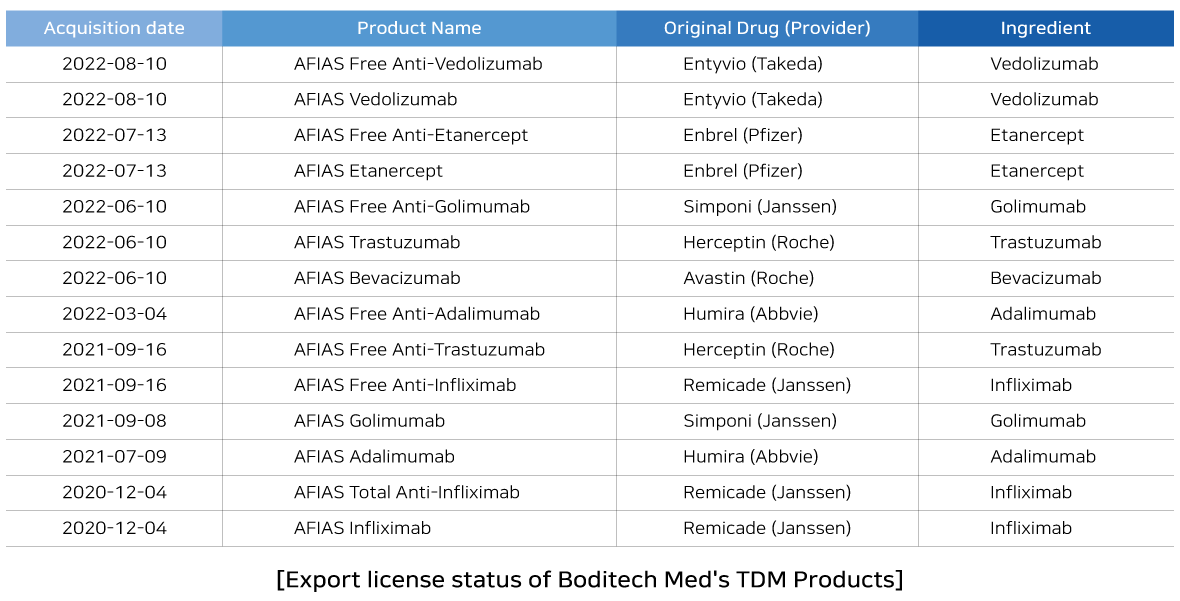
We are delighted to share the news that Boditech Med is accelerating TDM business and acquired additional export license for four TDM products such as ‘AFIAS Vedolizumab’, ‘AFIAS Free Anti-Vedolizumab’, 'AFIAS Etanercept' and 'AFIAS Free Anti-Etanercept' by MFDS. With this approval, Boditech Med secures 14 TDM products with export license.
Therapeutic drug monitoring (TDM) measures the concentration of drugs and ADA(Anti-drug antibody) formation in patients' blood in order to clarify whether the drug level is within a therapeutic range.
AFIAS Vedolizumab is the TDM product to measure the drug concentration of patients receiving Vedolizumab for moderate to severe ulcerative colitis or Crohn’s disease. AFIAS Free Anti-Vedolizumab is to confirm the presence of ADA as an immunogenic response to Vedolizumab.
AFIAS Etanercept is the TDM product to measure the drug concentration of patients receiving Etanercept for rheumatoid arthritis, Psoriatic arthritis, Ankylosing spondylitis, and Plaque psoriasis. AFIAS Free Anti- Etanercept is to confirm the presence of ADA as an immunogenic response to Vedolizumab.
It is expected that the needs of Entyvio and Enbrel will increase as the increase in the indication and in the number of patients. In addition, the incidence of inflammatory disease has been increasing rapidly due to an aging population which may also increase the demand of TDM products.
The Boditech Med’s TDM products take only 12 mins to get a result, which is very competent as other TDM products take up to three weeks. And the products offer competitive performance compared to lab devices and strengthen user convenience.
It is undeniable that the global therapeutic monoclonal antibody market is a burgeoning market. According to the MarketsandMarkets Research, the TDM market is expected to grow from $2.04 billion in 2020 to $2.6 billion in 2026 at a CAGR of 9.4%.
Boditech Med has been striving to secure a new long-term growth plan with TDM products. Boditech Med obtained export licenses for a total of 14 TDM products for autoimmune and oncology drugs, starting with the export license approval for 'AFIAS Infliximab' in December 2020.
Eui-Yul Choi, CEO of Boditech Med said, “We are accelerating TDM business with this additional approval of the export license. We will drive our business with aggressive marketing promotions and securing diverse distribution channels in order to become a market leader in the TDM market.”

