Boditech Med is selected as one of the agencies for the national bio industry project by the MOTIE
- Collaboration with LyseNTech, Seoul National University Bundang Hospital, The University of Sydney, and KBIO HEALTH (Osong Medical Innovation Foundation)
- Planning to take a lead in the inhalable drugs market with our nebulizer entitled “SnycNeb” as a domestic company
We are delighted to share the news that Boditech Med is selected as one of the agencies for the national bio industry project, which is operated by the Ministry of Trade, Industry and Energy (MOTIE). The project is to encourage and support the development of biotechnology for future business and to strengthen the main industry’s competitiveness of South Korea. The Ministry of Trade, Industry and Energy will support $29.9 million in total, including $19.1 million for the Bio Industry.
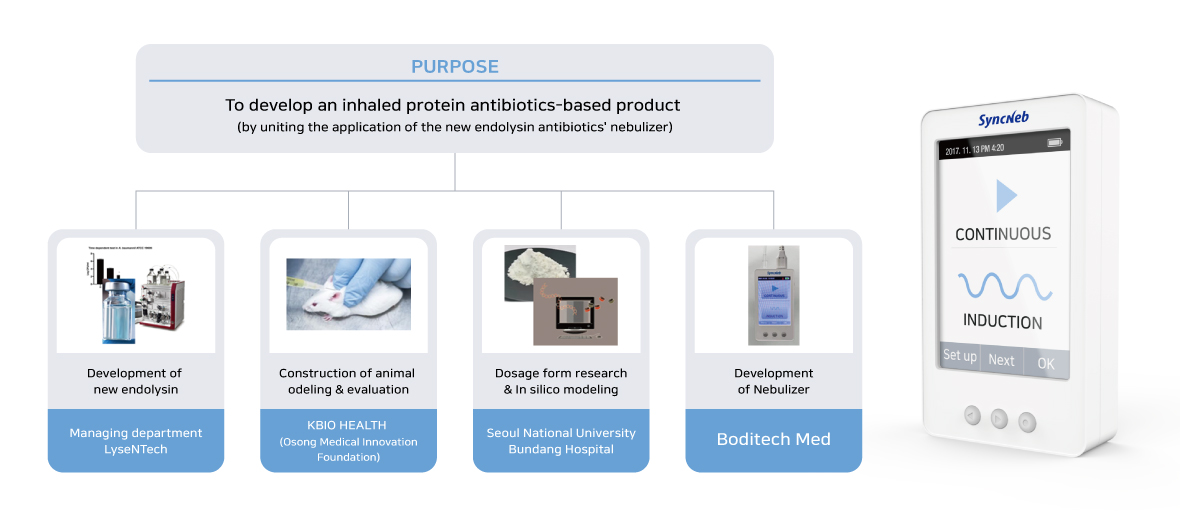
Boditech Med will be working with LyseNTech, Seoul National University Bundang Hospital, The University of Sydney, and KBIO HEALTH (Osong Medical Innovation Foundation).
For this joint research project, LyseNTech will work on developing a new antibacterial substance entitled “Endolysin”. Seoul National University Bundang Hospital and The University of Sydney will focus on developing ultimate pharmaceutical dosage forms for the nebulizer. And KBIO HEALTH (Osong Medical Innovation Foundation) will be in charge of the construction of animal modeling and will appraise the validity of its application.
Boditech Med will develop a commercial nebulizer considering several factors such as the particle size of the drug, the velocity of the ultrasonic vibrator, the viscosity for liquefaction, the vibration intensity, and suitable temperature for usage to finally deliver the drug to the lung safely. In the 3rd year of this project, it is expected that Boditech Med is planning to expand its patient group into general patients by developing a portable mouthpiece.
The Boditech Med’s nebulizer entitled ‘SnycNeb’, based on vibrating mesh technology, could adjust the amount of drug in less than 5 μm. Thus, it could deliver the drug more efficiently to the patient’s lung than ‘Jet type’ nebulizer, which is mostly used in ICU(Intensive Care Unit). And it is expected that it will be used more conveniently as it makes less noise.
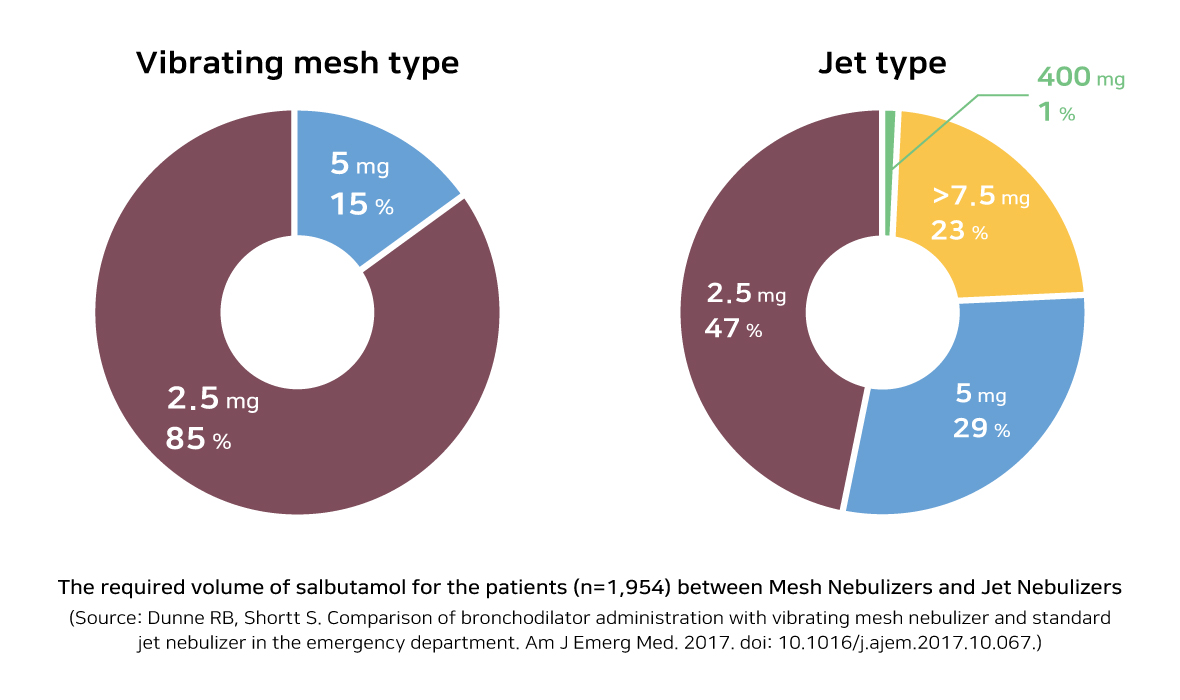
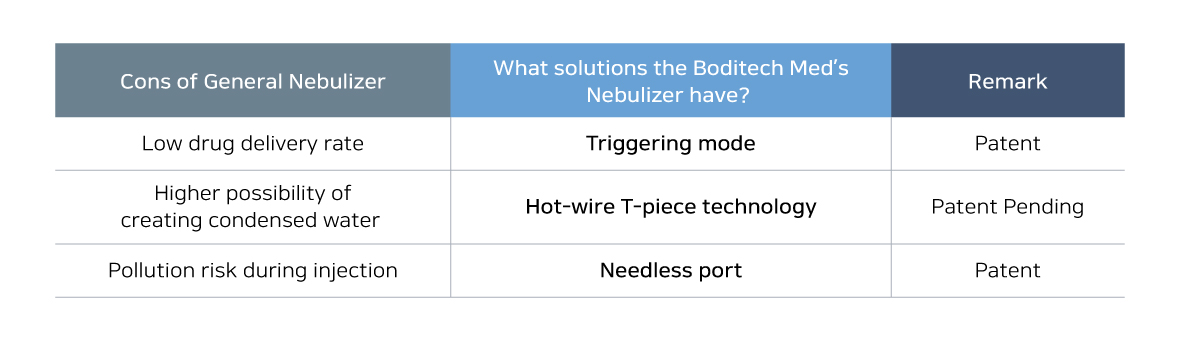
As the previous nebulizer is designed to spray its drug continuously not considering when the patients inhale or exhale, the delivery rate of the drug is low because the drug could be only delivered to the lung when the patient inhales. And when the patient exhales, there is high possibility that other patients and medical staffs who are not being prescribed could get affected by the drugs. To solve this problem, Boditech Med developed “Triggering mode” that appropriately spray its drug when the patient inhale only. It will prevent wasting of the drug usage and will maximize its performance.
In addition, based on the hot-wire T-piece technology, which is patent pending, will be applied to minimize the condensed water problem that might be increase the risk of infection when patient is put on a respirator. And the other risk such as pollution that could occur when the patient’s breathing circuits are opened for drug injection will be minimized with needle free injection technology. The SyncNeb is on the New Health Technology Assessment and Boditech Med is planning to acquire domestic use of SyncNeb by Ministry of Fodd and Drug Safety (MFDS) in the latter half of this year.
Inhalation-type therapeutics are devices and drugs that use an inhaler to deliver a certain amount of drug directly to the lungs. It is essential for the treatment of respiratory diseases such as asthma and COPD (chronic obstructive pulmonary disease). On the other hand, invasive injections, mainly used in the past, have a low delivery rate to the lungs and are difficult for patients to self-administer.
The global pharmaceutical companies dominated the global inhalable drugs market, which is estimated to be worth $30.9 billion globally and $231 million in the domestic market. Even though there are no patent issues, most pharmaceutical companies couldn’t get into the market because of the high technical barriers of the inhaler device. The nebulizer should be able to adjust the amount of the drug in micrograms (μg, one-million of a gram).
Eui-Yul Choi, CEO of Boditech Med said, “There are few companies who are getting into the inhalable drugs market at this moment. Through this national bio industry project, we will break down technical barriers with our innovative product “SyncNeb” taking lead in this market.”

