Boditech Med has obtained the export license of COVID-19 Ag Saliva rapid test kit
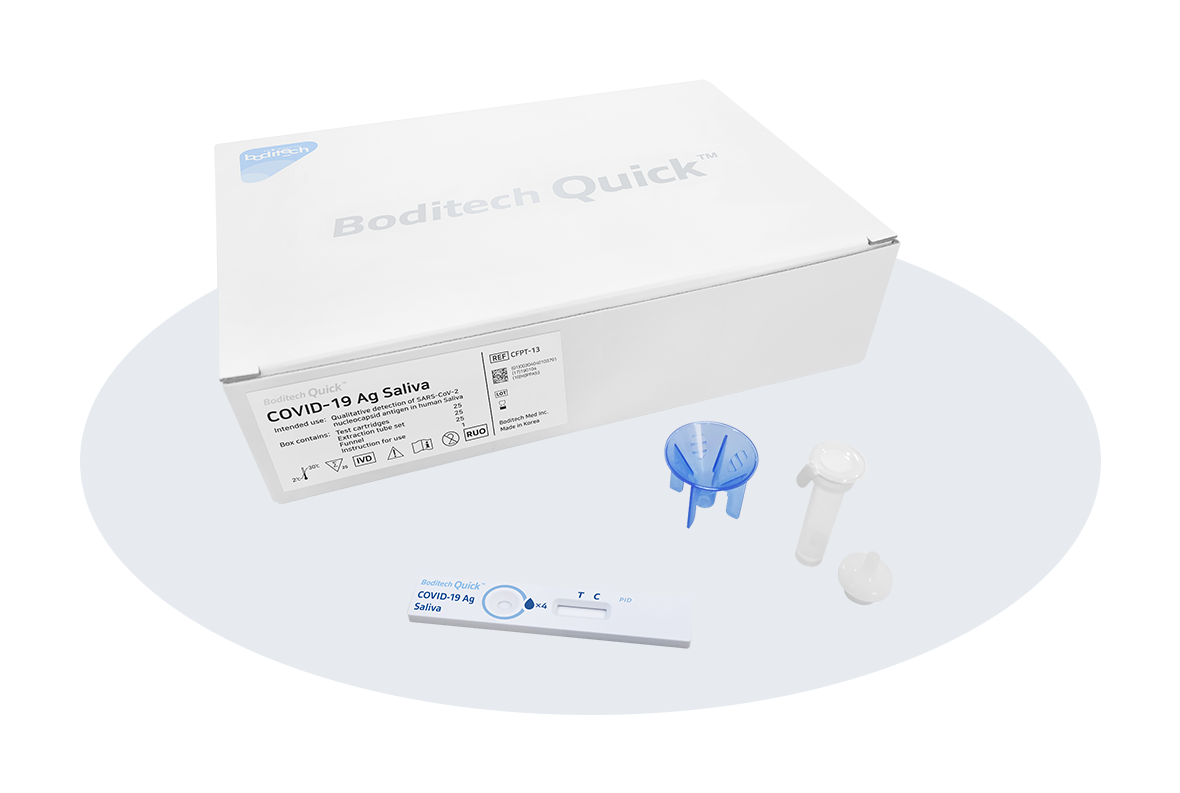
- Enabling detect the COVID-19 infection with saliva sample in 15 minutes
- Painless and convenient sampling process using saliva without putting the swab into the nose
- Optimization for the diagnosis of children and teenagers in the With-COVID-19 Era
- Under the development of various Therapeutic Drug Monitoring diagnostic solutions for therapeutic antibodies and anti-cancer drugs to target the global companion diagnostics market
We are pleased to announce that Boditech Med has obtained the export license with rapid-testing for COVID-19 antigen testing using saliva that can enhance the convenience of testing within 15 minutes for the COVID-19 infection. For this approval, it is highly expected that Boditech Med will acquire the domestic use of self-testing of COVID-19 antigen in the near future.
The Boditech Quick™ COVID-19 Ag Saliva has more than doubled its performance (sensitivity 80%, specificity 100%) compared the existing export product. It met the requirement of the Ministry of Food and Drug Safety standards with a sensitivity of 90% and a specificity of 100%. Based on easy-to-use operation, it also reduces the manufacturing cost by more than half compared to existing products. Thus, the demand of the Boditech Quick™ COVID-19 Ag Saliva is expected to increase in South Korea and abroad.
Boditech Quick™ COVID-19 Ag Saliva is ideally optimized for user convenience. As the users can collect their test samples from their mouth and all the users need to do is to drop the saliva sample into the extraction buffer tube, the test procedure is much simpler than existing kits which can detect COVID-19 infection by putting the swab into the nose. Especially, as it doesn’t need to put the swab in your nose, the users are free of pain with simplified test procedure and easy-to-use operation. For entering to the With-COVID-19 Era, the usage of the COVID-19 Ag test is expected to continuously increase for the next few years. It indicates that Boditech Quick™ COVID-19 Ag Saliva could be ultimate solution for children, teenagers, and elderly people who used to have resistance to collect samples through their nose.
Additionally, COVID-19 variants such as BA.1(Omicron) and BA.2(Stealth Omicron) have a strong tendency to infect the upper respiratory tract while not to enter inside the human body. Therefore, saliva sample collection for COVID-19 antigen detection could be better than that of the nasopharyngeal swab.
Eui-Yul Choi, CEO of Boditech Med said, “Boditech Quick™ COVID-19 Ag Saliva has attracted a lot of interest in many countries, as it has a simplified test procedure with easy-to-use operation and presents higher sensitivity and specificity regarding the major COVID-19 variants such as Omicron and Stealth Omicron comparing existing RDT products". He continued, “It is expected that we can acquire the approval for domestic use of Boditech Quick™ COVID-19 Ag Saliva by the Korean Ministry of Food and Drug Satefy in the near future as we successfully obtained the export license”

