Boditech Med has obtained domestic use approval of Boditech Quick™ COVID-19 Ag Saliva from the MFDS
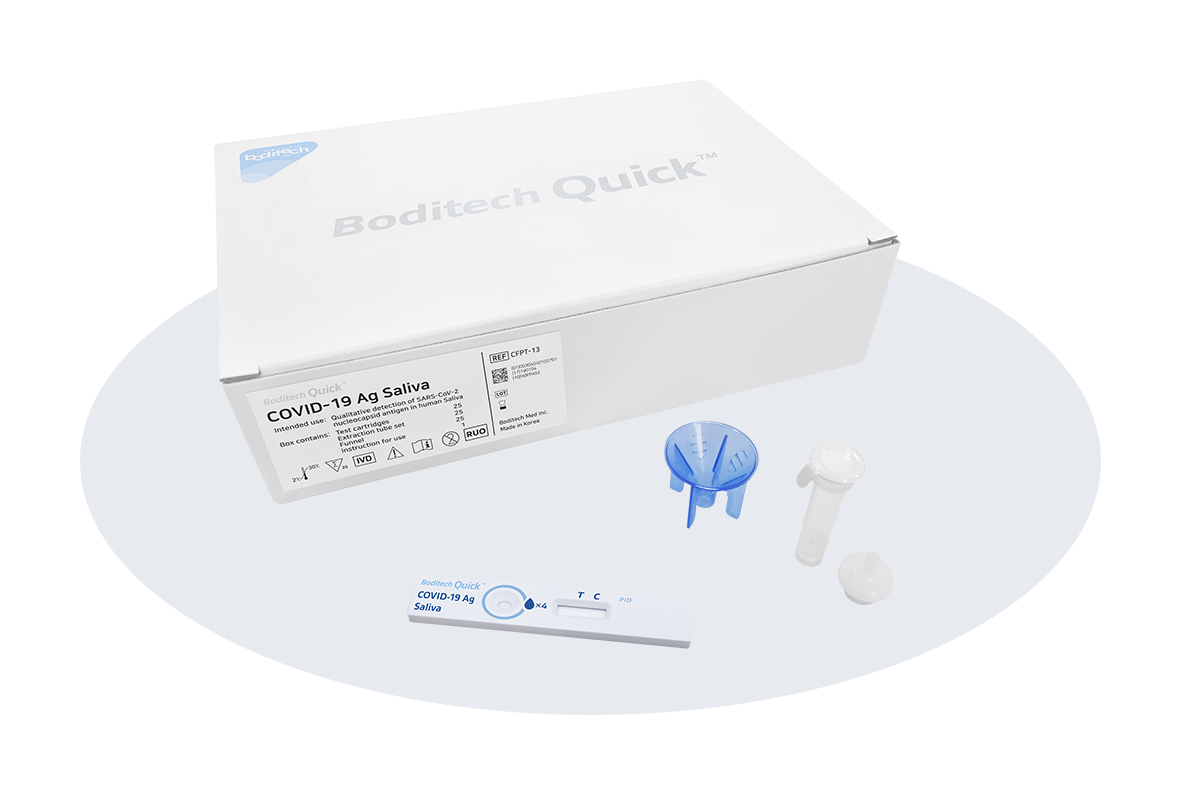
- The most advanced COVID-19 Ag home test with saliva sample (sensitivity 93.8%, specificity 99.5%)
- Users can get a test result in 15 minutes
- It can detect COVID-19 variants including the Omicron variant
We are excited to announce that Boditech Med has obtained domestic use approval of Boditech Quick™ COVID-19 Ag Saliva from the Ministry of Food and Drug Safety. It can determine whether or not you have been infected with COVID-19 within 15 minutes using saliva sample.
Boditech Quick™ COVID-19 Ag saliva has the best performance compared to any other existing COVID-19 test kits with saliva sample in the South Korea. Resulting of the clinical evaluation from 281 volunteers, the performance shows a clinical sensitivity of 93.8% and a clinical specificity of 99.5%. The sensitivity of 93.8% is the best performance among other products obtained domestic use license.
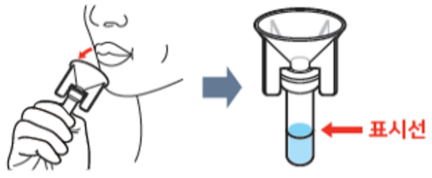
Boditech Quick™ COVID-19 Ag Saliva has a test procedure of spitting saliva collected from the throat by coughing up to the line of the extraction buffer tube, mixing it with the buffer in the tube, and dropping it on the test cartridge. Test results can be identified within 15 minutes.
It also can detect COVID-19 variants. As a result of the variants test, it is possible to detect all the variants identified so far, including the Omicron variant BA.5. According to the Korea Centers for Disease Control and Prevention (KCDC), it reported that BA.5, BF7, and BQ.1 accounted for 88.3%, 2.2%, and 1% of total COVID-19 cases in the 4th week of October.
As Omicron variants infect the upper respiratory tract and have a strong property of not entering deep into the body, a sample collection using saliva may be more useful for the detection of the COVID-19 antigen than using the existing nasopharyngeal sample collection.
The saliva self-test kit is evaluated as the best product for children and adolescents who are reluctant to pierce their noses as they can use it easily and conveniently. Authorities are increasing the distribution of COVID-19 saliva self-test kits, especially in educational institutions.
Since the second semester of 2022, the Seoul Metropolitan Government has been stockpiling saliva self-test kits at the Seoul Metropolitan Office of Education distributing them to schools and students who need them. The Jeju Special Self-Governing Provincial Office of Education is also distributing self-test kits using saliva sample considering the difficulties of preschool and elementary school students in testing.
This approval is expected to boost exports of Boditech Quick™ COVID-19 Ag Saliva. In fact, it is known that some Southeast Asian countries require the approval for domestic use from the Korean Ministry of Food and Drug Safety separately for getting the export license.
In addition, as Boditech Quick™ COVID-19 Ag Saliva is the first over-the-counter (OTC) product, Boditech Med is planning to actively target major overseas OTC markets including Korea. Boditech Med began to supply Rapid Diagnostic Kit overseas at the end of last year.
Eui-Yul Choi, CEO of Boditech Med said, “The approval of Boditech Quick™ COVID-19 Ag Saliva has the best performance compared to existing products”. He also mentioned, "It will be a very valuable diagnostic kit for the elderly and children as well as primary medical institutions and emergency rooms as it can detect all the variants of COVID-19 so far.". He emphasized "As the reliability on this product is increasing worldwide, we are planning to actively export this product."